Flowchart of included drugs. EMA, European Medicines Agency; FDA, Food... | Download Scientific Diagram

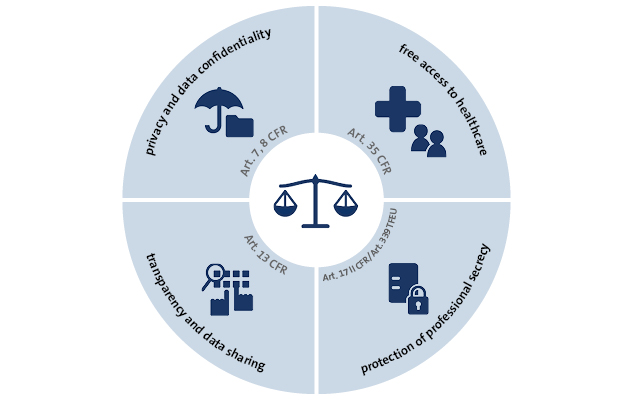
Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States - Cytotherapy

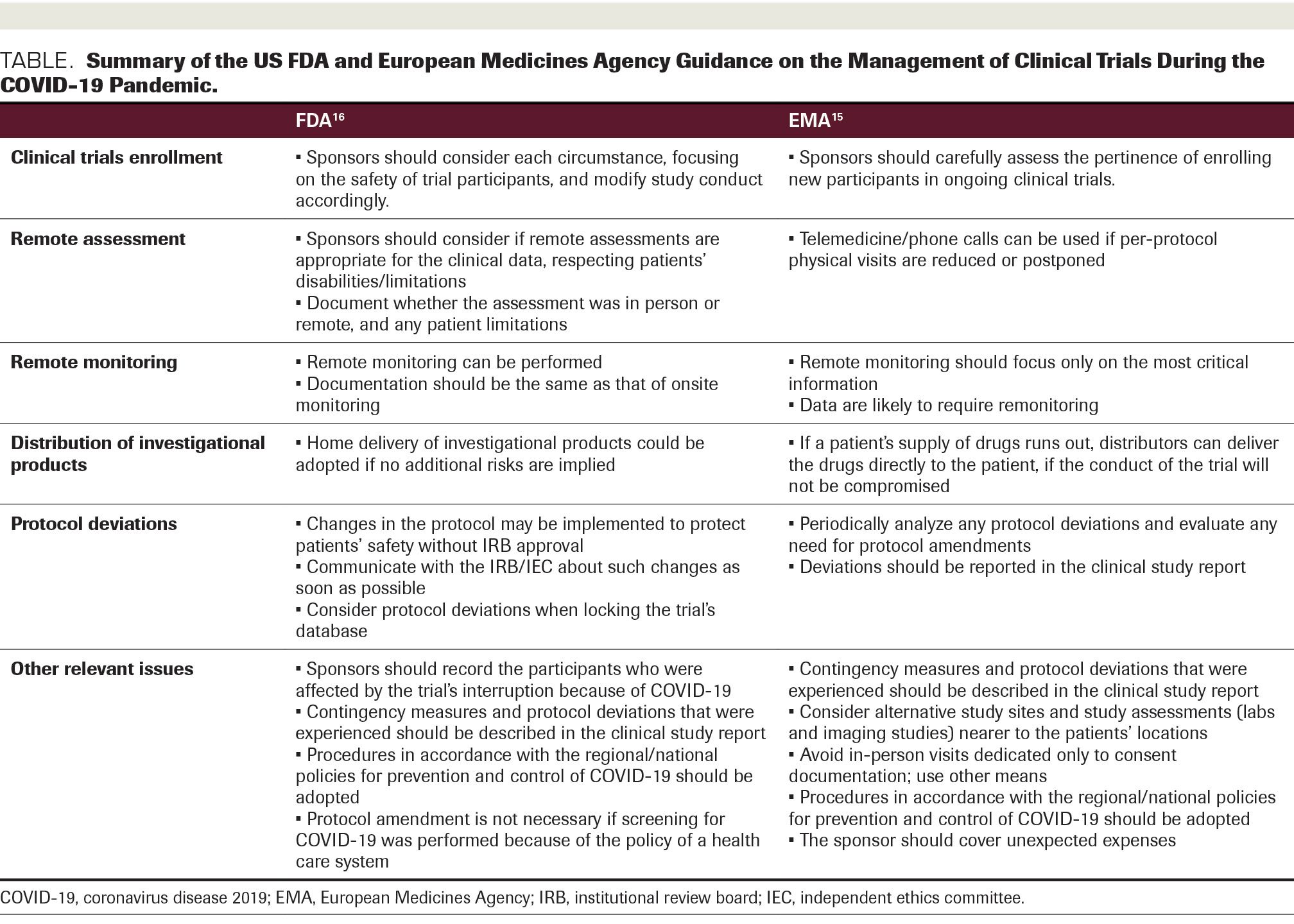
Assessment of the Regulatory Dialogue Between Pharmaceutical Companies and the European Medicines Agency on the Choice of Noninferiority Margins - Clinical Therapeutics

Characteristics of Single Pivotal Trials Supporting Regulatory Approvals of Novel Non‐orphan, Non‐oncology Drugs in the European Union and United States from 2012−2016 - Morant - 2019 - Clinical and Translational Science - Wiley Online Library
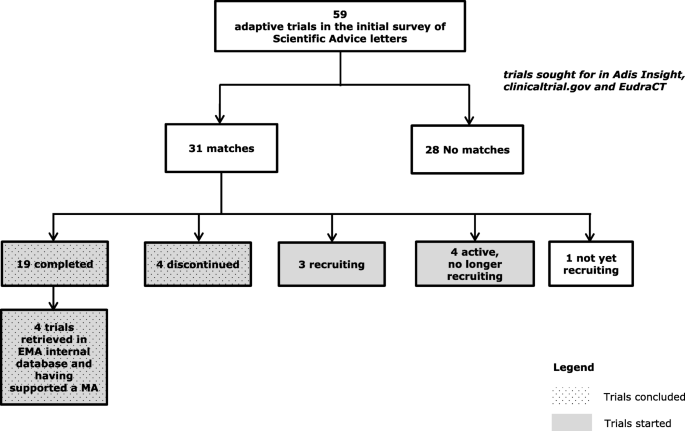
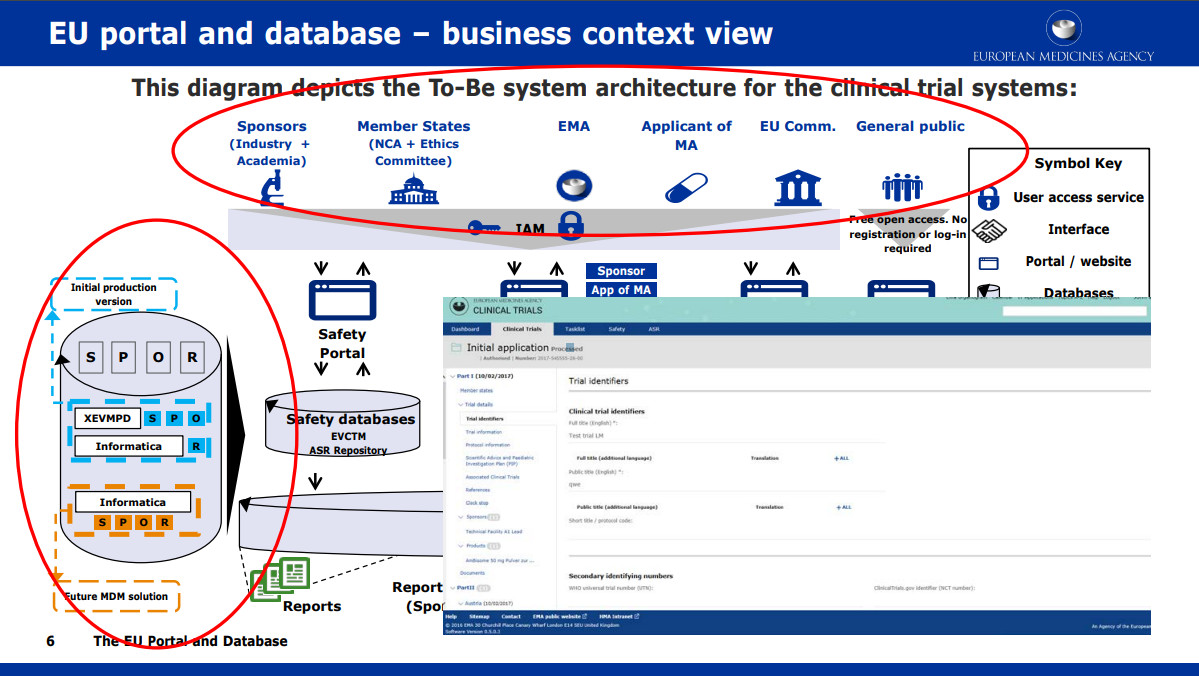
Adaptive designs in clinical trials: from scientific advice to marketing authorisation to the European Medicine Agency | Trials | Full Text