PDF) Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine
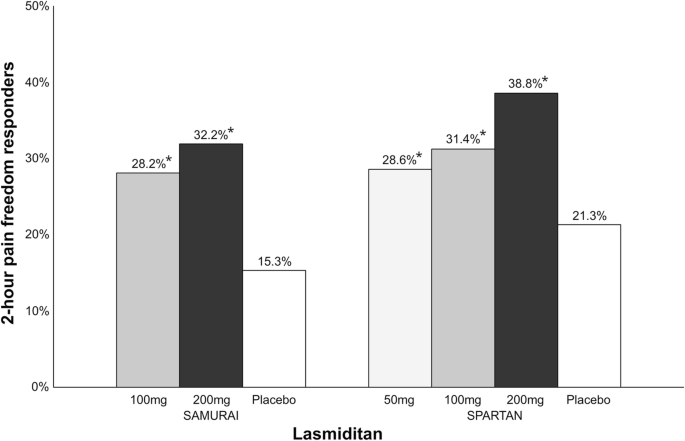
H8H-CD-LAHK A Study of Three Doses of Lasmiditan (50 mg, 100 mg and 200 mg) Compared to Placebo in the Acute TReaTment of MigrAi
Therapeutic novelties in migraine: new drugs, new hope? | The Journal of Headache and Pain | Full Text

Phase 2 randomized placebo‐controlled study of lasmiditan for the acute treatment of migraine in Japanese patients - Sakai - 2021 - Headache: The Journal of Head and Face Pain - Wiley Online Library

Tolerability and Safety of Lasmiditan Treatment in Elderly Patients With Migraine: Post Hoc Analyses From Randomized Studies - Clinical Therapeutics
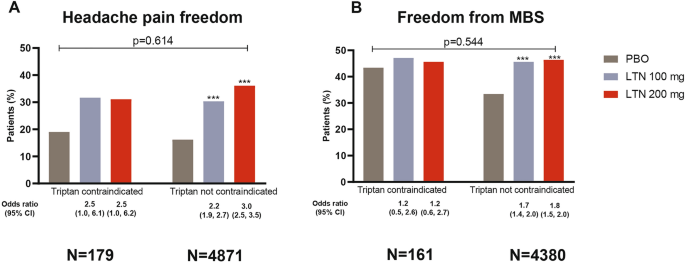
Lasmiditan for Patients with Migraine and Contraindications to Triptans: A Post Hoc Analysis | SpringerLink

Lasmiditan Col144 Investigational Drug Treatment Acute Stock Vector (Royalty Free) 1332679202 | Shutterstock

Rational Use of Lasmiditan for Acute Migraine Treatment in Adults: A Narrative Review - Clinical Therapeutics
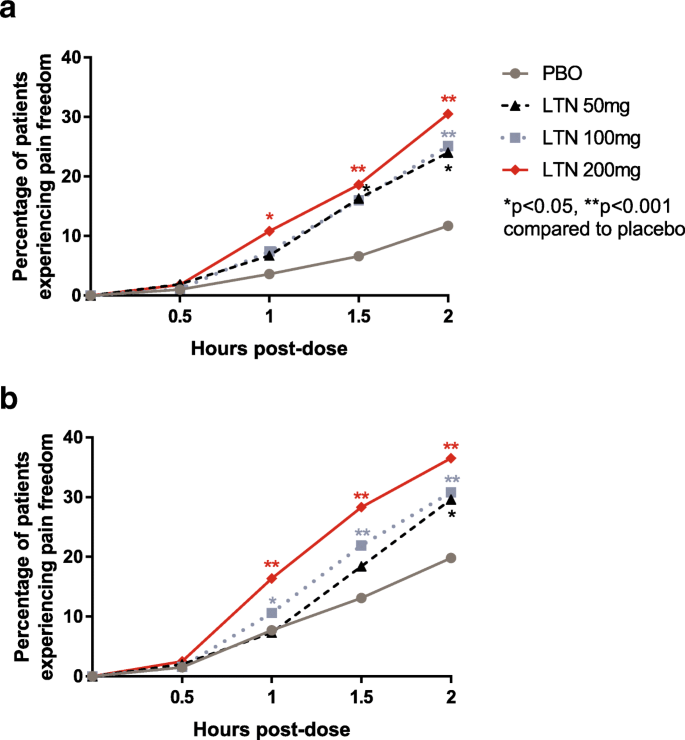
Efficacy and safety of lasmiditan in patients using concomitant migraine preventive medications: findings from SAMURAI and SPARTAN, two randomized phase 3 trials | The Journal of Headache and Pain | Full Text

Efficacy and tolerability of lasmiditan, an oral 5-HT1F receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo- controlled, parallel-group, dose-ranging study - The Lancet Neurology
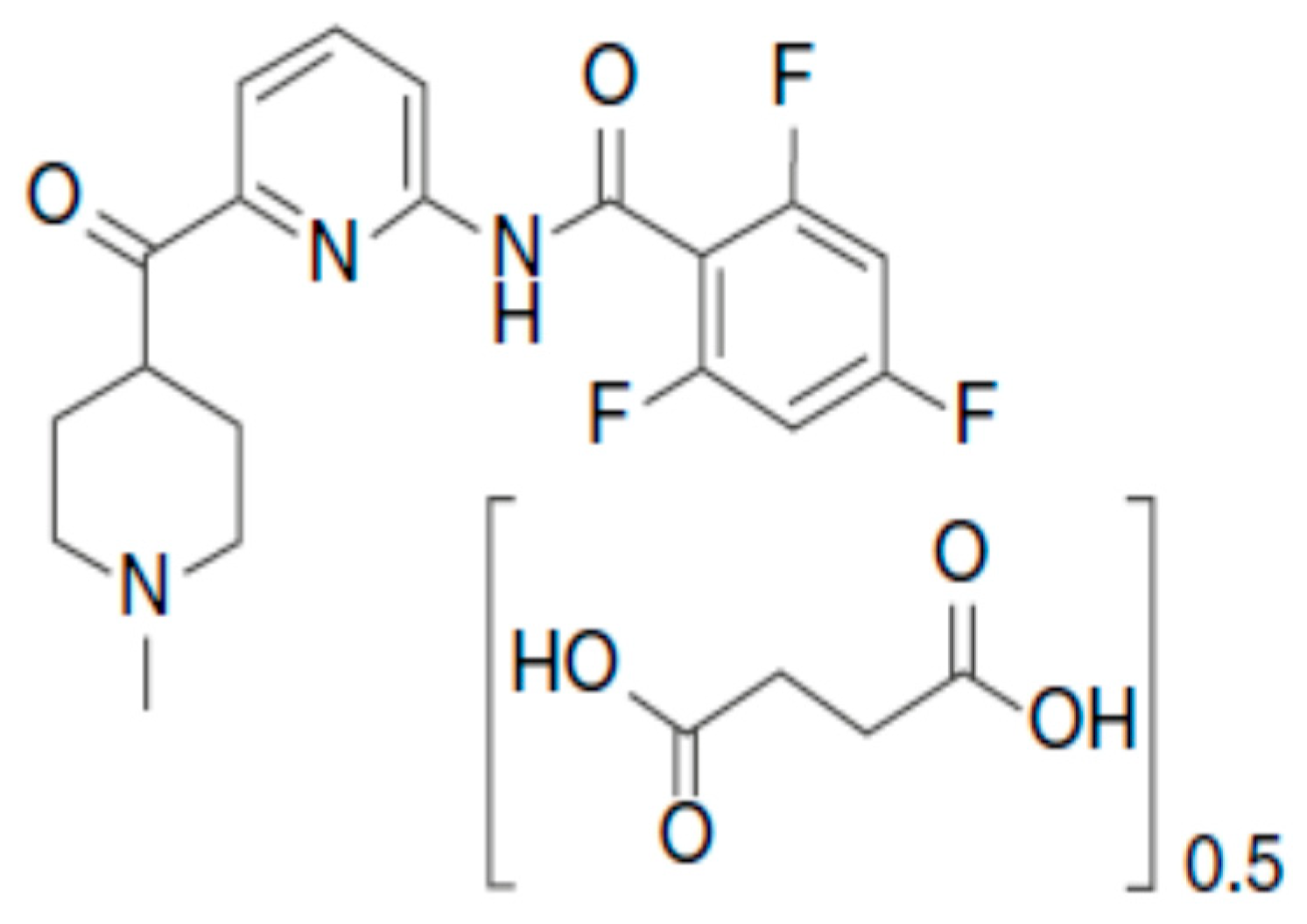
Pharmaceutics | Free Full-Text | Pharmacokinetics, Pharmacodynamics and Drug –Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies | HTML

PDF) Sustained responses to lasmiditan: Results from post-hoc analyses of two Phase 3 randomized clinical trials for acute treatment of migraine
The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migr
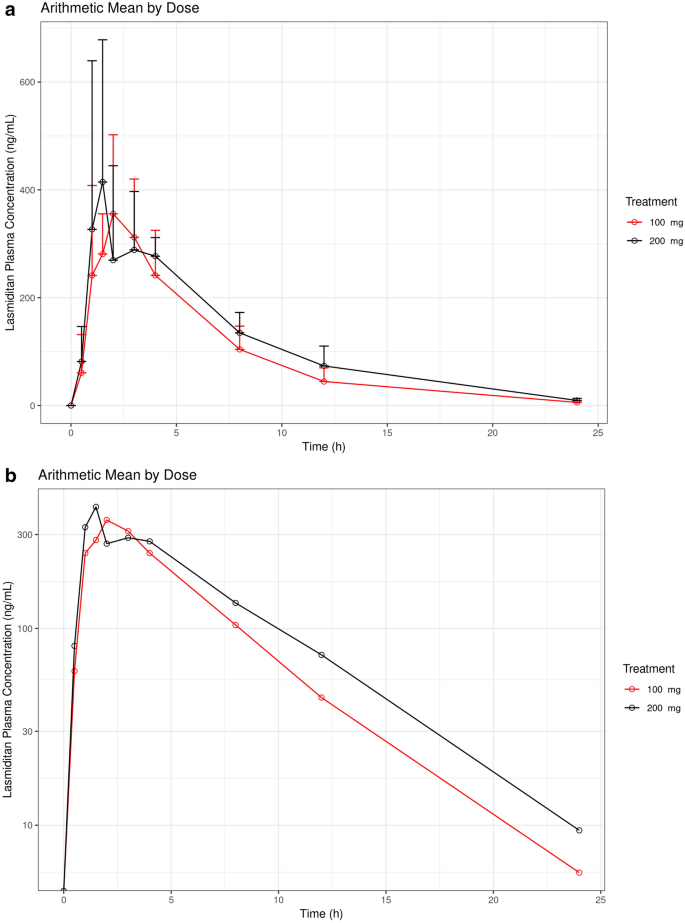
Protocol H8H-CD-LAHN (V1) (CUD-P4-001 (COL MIG-113)) A Phase I, Multicenter, Open-Label, Parallel-Group Adaptive Pharmacokinetic