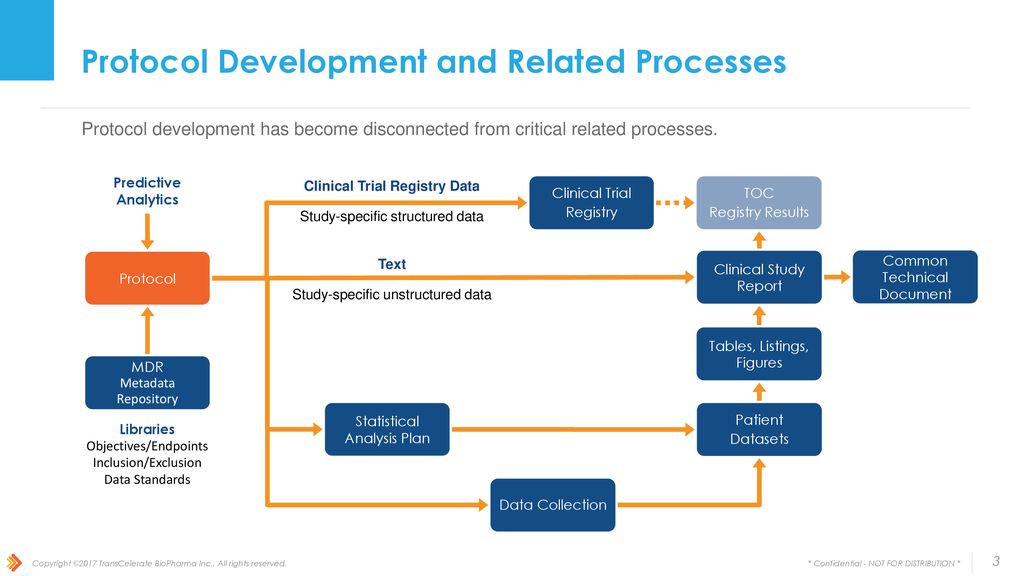
Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - Clinical Therapeutics
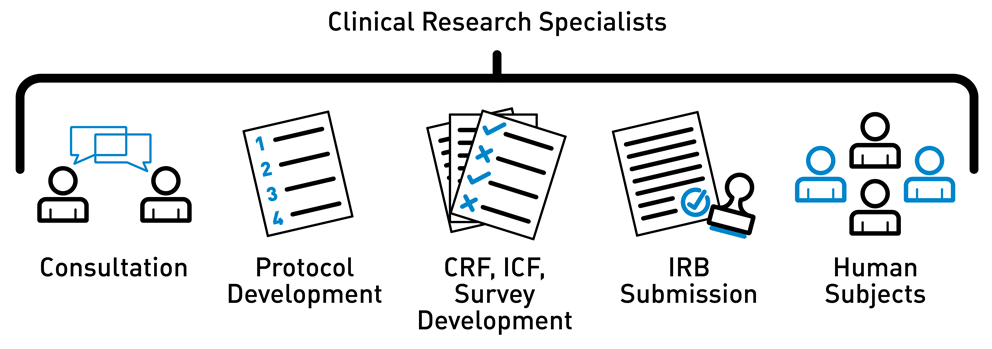
Clinical Research Development assists with various administrative aspects of the IRB and human subject trials and materials.

Figure 1 from Steps and time to process clinical trials at the Cancer Therapy Evaluation Program. | Semantic Scholar

11/11/04 Clinical Research and Development in the Pharmaceutical and Biotechnology Industry Robert Anderson, MHA, CCRA, CCRCP Director, Clinical Trials. - ppt download
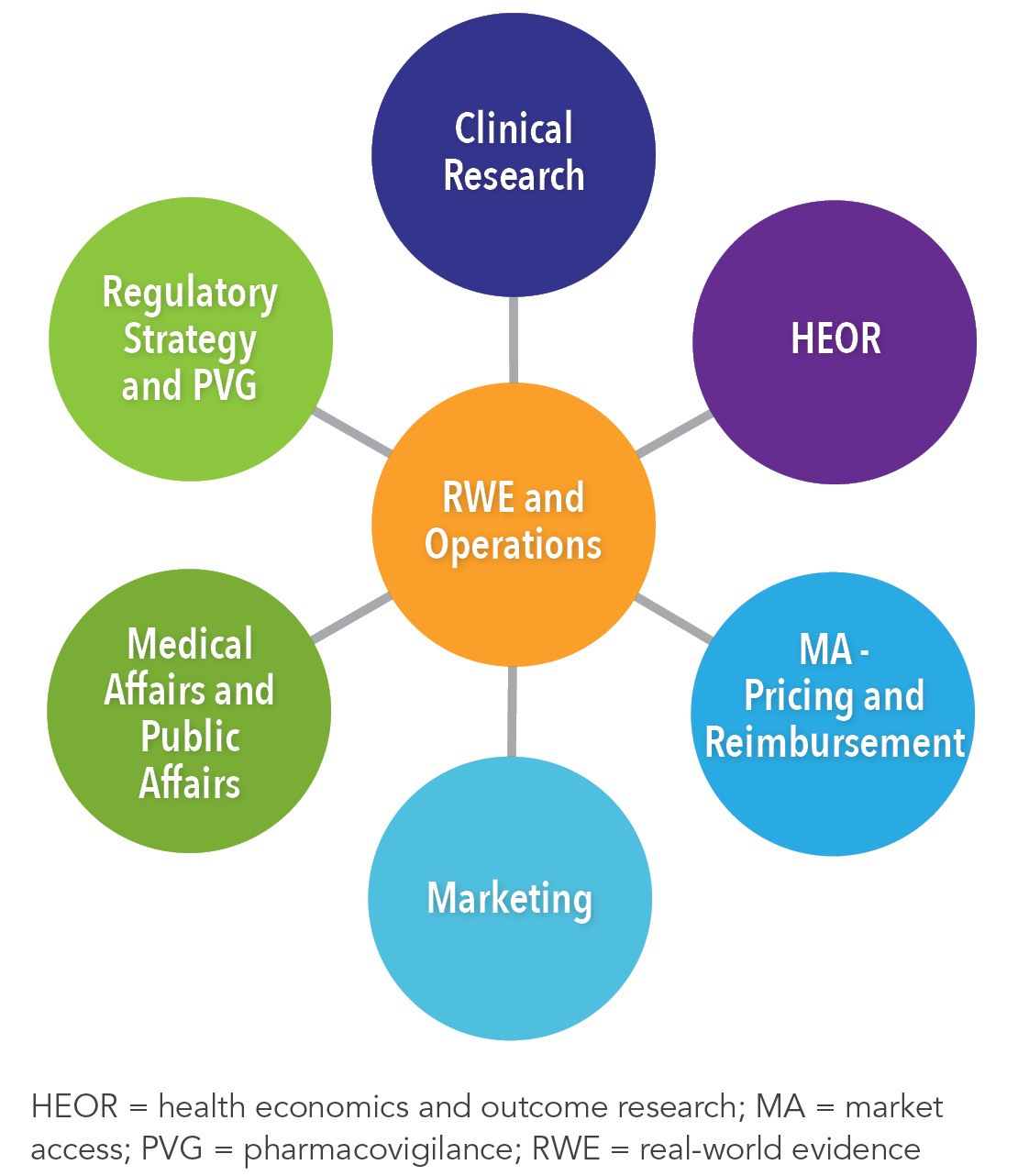
White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera
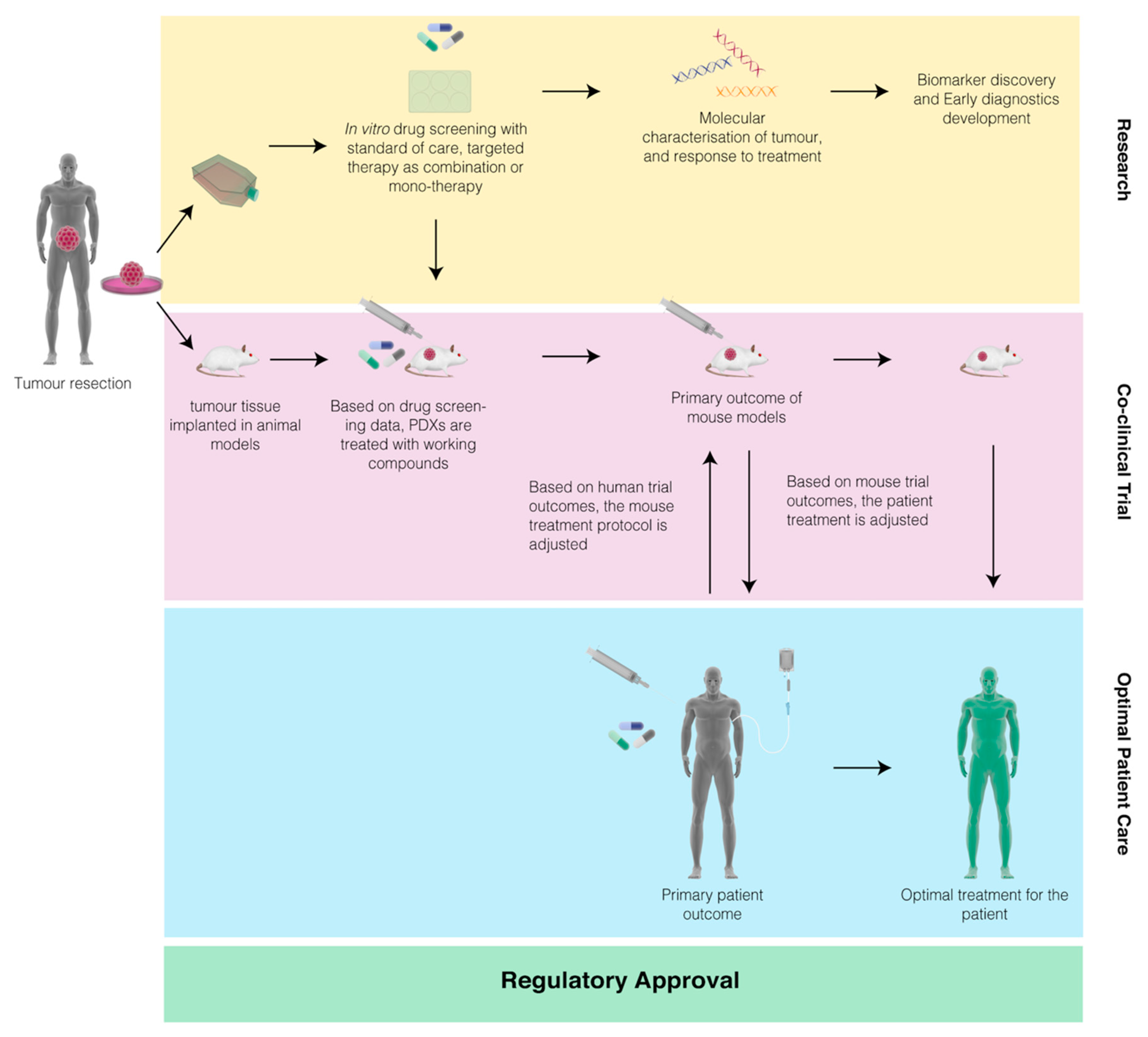
Pharmaceuticals | Free Full-Text | Co-Clinical Trials: An Innovative Drug Development Platform for Cholangiocarcinoma